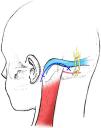
Suboccipital lateral approach is a common practice in neurosurgery to expose the region of the cerebellopontine angle. Postcraniectomy headache (PCH) is one of the most frequent complications that diminish the quality of life of patients.
ObjectiveTo compare postcraniectomy pain in patients operated on for vestibular neurinomas by a suboccipital lateral approach by 2 different incisions.
Material and methodsProspective randomised research study. Follow-up of patients operated for vestibular neurinomas between July 2017 and May 2019 (n=40) by the same surgeon. One group received the classical linear incision (n=20) and another group the alternative incision in an inverted “U” (modified Dandy) (n=20). Pain intensity was evaluated by numerical scale. A minimum follow-up of 3 months was carried out. The impact on quality of life was measured by the SF-36 questionnaire short version both before and after surgery.
ResultsThe average age was 46.1 years. The overall PCH index was 27.5% (n=11) of the patients. The incidence of pain in the group that received the classical incision (A) was 20% (n=4) and in group B was 35% (n=7).
ConclusionsWe found a higher rate of post-craniectomy headache in patients who underwent a “modified Dandy” incision. These are preliminary data of an undergoing study and we hope to obtain more representative information in the future. We recommend interdisciplinary follow up for the integral treatment of PCH.
El abordaje suboccipital lateral es de práctica habitual en neurocirugía para exponer la región del ángulo pontocerebeloso. El dolor poscraniectomía (DPC) es una de las complicaciones más frecuentes que disminuyen la calidad de vida de los pacientes.
ObjetivoComparar el DPC en pacientes operados de neurinomas vestibulares por un abordaje suboccipital lateral mediante 2 incisiones distintas.
Material y métodosEstudio de investigación prospectivo aleatorizado. Se realizó seguimiento de un grupo de pacientes operados por neurinomas vestibulares entre julio de 2017 y mayo de 2019 (n=40) por un mismo cirujano. Un grupo recibió la incisión lineal clásica (n=20) y otro grupo la incisión alternativa en «U» invertida o «Dandy modificada» (n=20). La intensidad del dolor fue evaluada mediante escala numérica. Se realizó un seguimiento mínimo de 3 meses. El impacto en la calidad de vida se objetivó mediante cuestionario SF-36 versión corta tanto pre- como posquirúrgico.
ResultadosLa edad promedio fue 46,1 años. El índice global de DPC fue del 27,5% (n=11) de los pacientes. La incidencia de cefalea en el grupo que recibió la incisión clásica (A) fue del 20% (n=4), en el grupo B fue del 35% (n=7).
ConclusiónEncontramos un mayor índice de DPC en los pacientes que recibieron una incisión tipo «Dandy modificada». Estos son datos preliminares de un estudio que continúa y esperamos obtener datos más representativos en el futuro. Recomendamos el seguimiento interdisciplinario para el tratamiento integral del DPC.
Article

If it is the first time you have accessed you can obtain your credentials by contacting Elsevier Spain in suscripciones@elsevier.com or by calling our Customer Service at902 88 87 40 if you are calling from Spain or at +34 932 418 800 (from 9 to 18h., GMT + 1) if you are calling outside of Spain.
If you already have your login data, please click here .
If you have forgotten your password you can you can recover it by clicking here and selecting the option ¿I have forgotten my password¿.