To analyze the change in the characteristics of presentation, evolution and treatment in the ICU, as well as the functional evolution at 12 months of spontaneous intracranial hemorrhages (ICHs) treated in an ICU reference center.
Patient and methodsDescriptive, retrospective study in a Neurocritical Reference Hospital. All admissions of patients with HICE during three periods are studied: 1999–2001 (I), 2015–2016 (II) and 2020–2021 (III). Evolution in the three periods of demographic variables, baseline characteristics of the patients, clinical variables and characteristics of bleeding, evolutionary data in the ICU are studied. At one year we assessed the GOS scale (Glasgow Outcome Score) according to whether they had a poor (GOS 1−3) or good (GOS 4−5) prognosis.
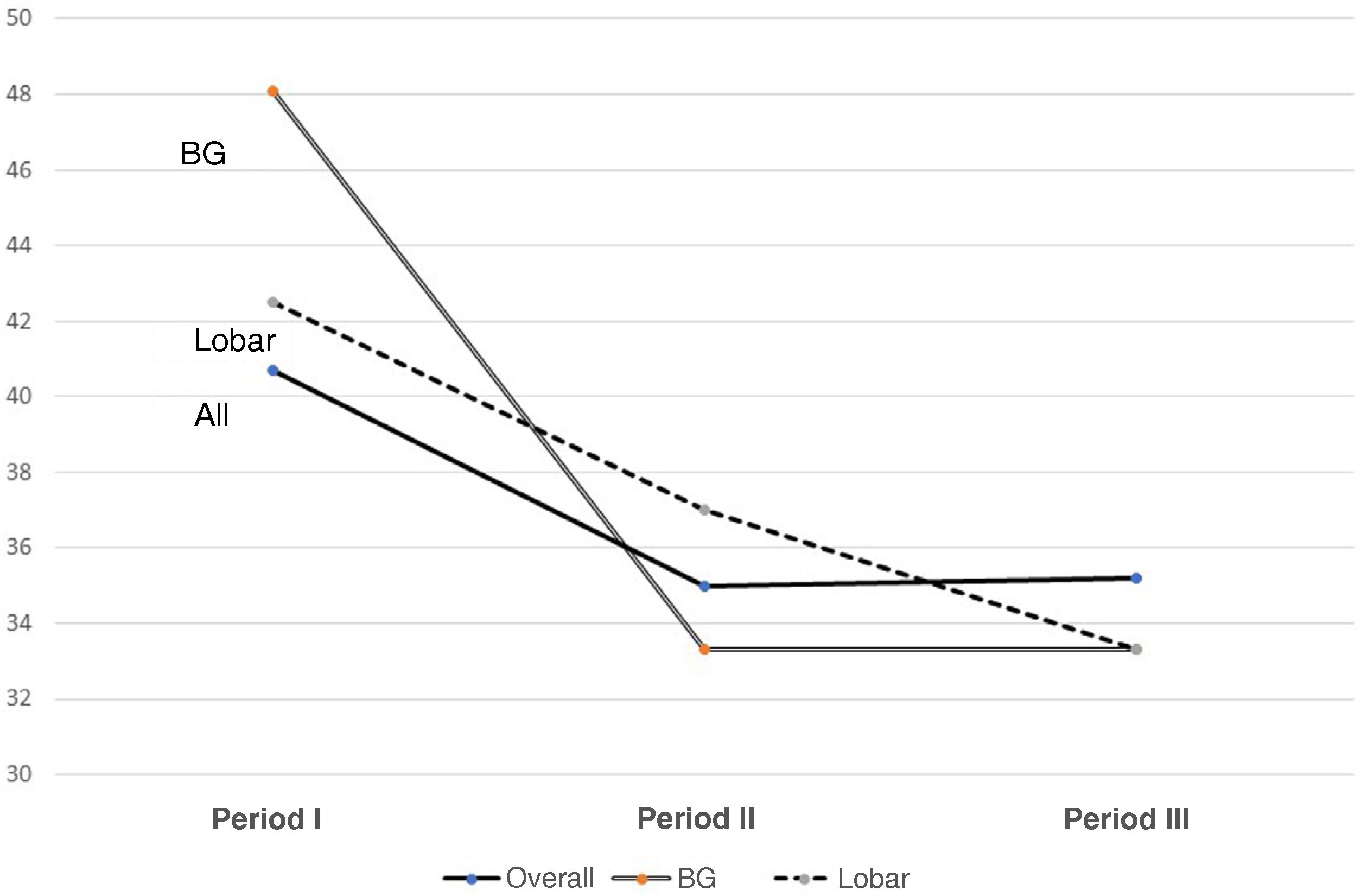
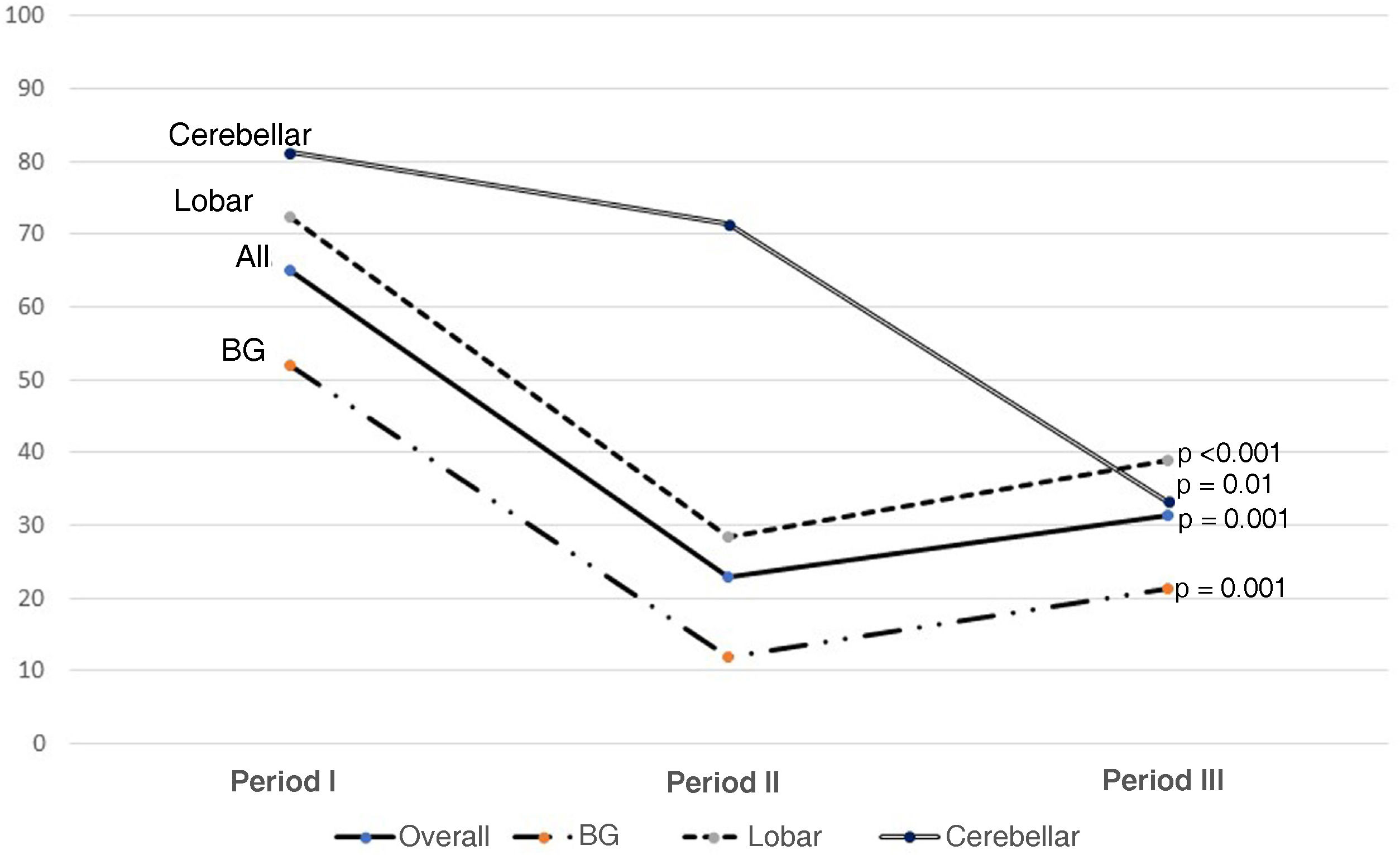
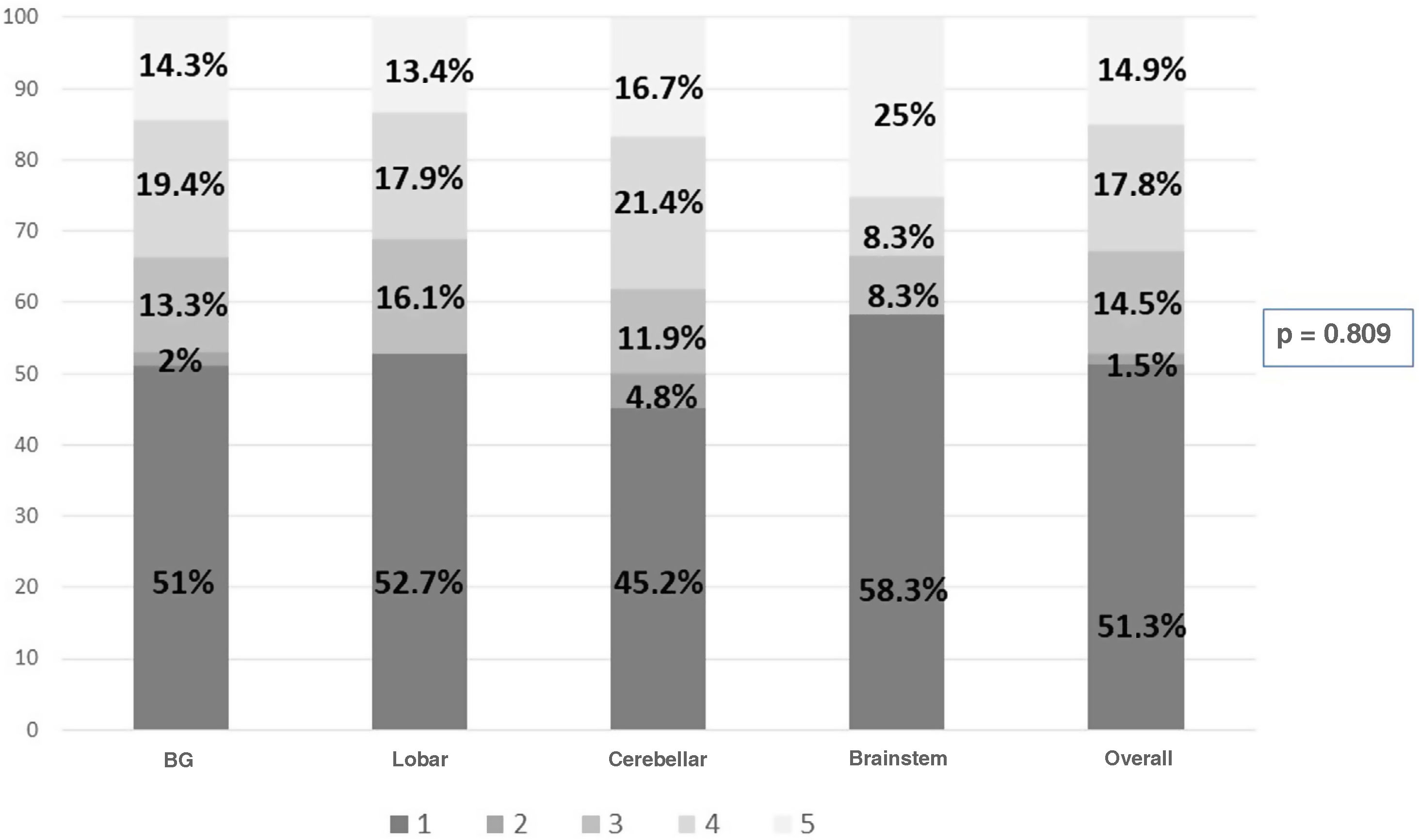
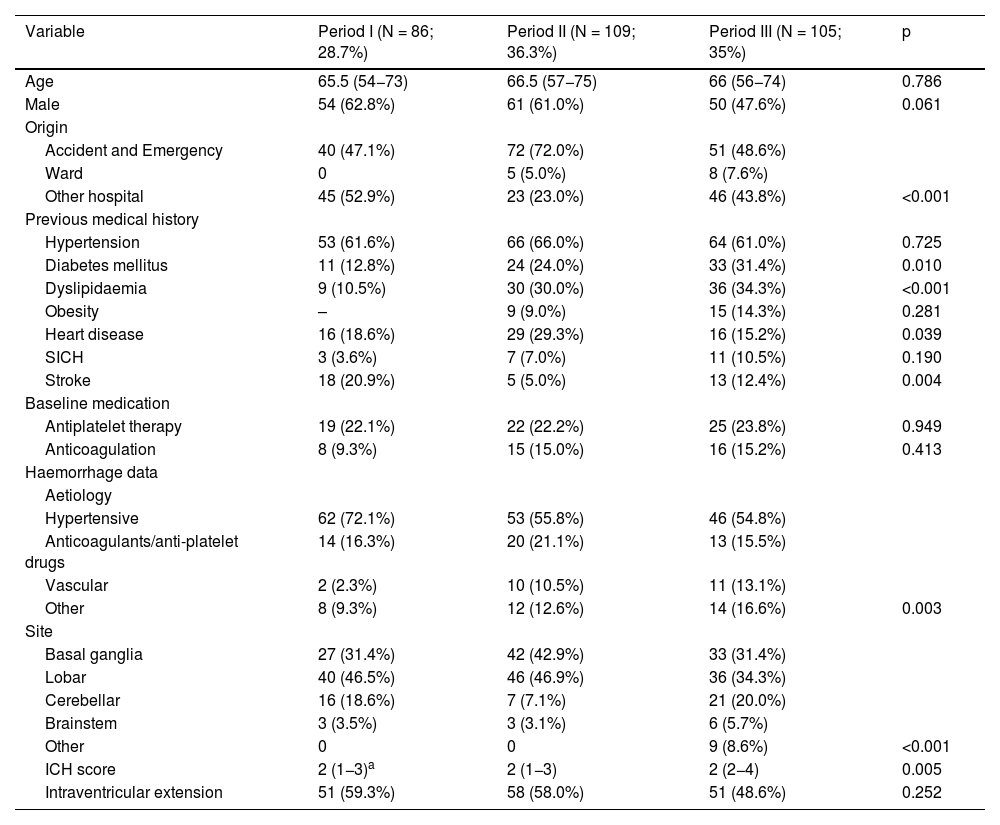
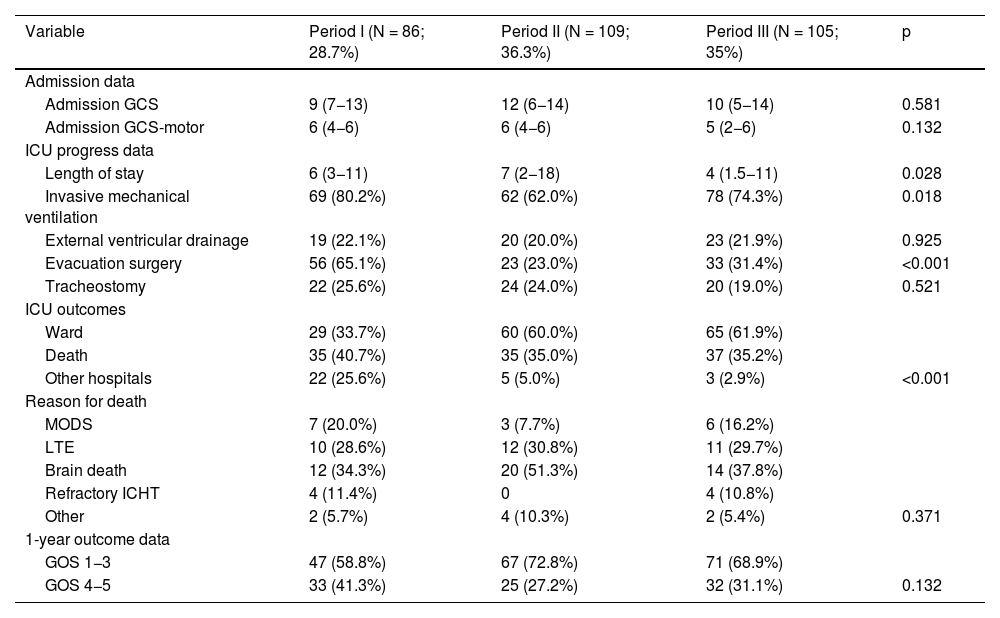
Results300 admitted patients, distributed in periods: I: 28.7%, II: 36.3% and III: 35%. 56.7% were males aged 66 (55.5–74) years; ICH score 2 (1−3). The ICU stay was 5 (2–14) days with a mortality of 36.8%. GOS 1−3 a year in 67.3% and GOS 4−5 in 32.7%. Comparing the three periods, we observed a higher prevalence in women, and the presence of cardiovascular factors; no changes in etiology; in relation to the location, it increases cerebellar hemorrhage and in the brainstem. Although the severity was greater, the stay in the ICU, the use of invasive mechanical ventilation and tracheostomy were lower. Open surgery has decreased its use by 50%. Mortality continues to be high, stagnating in the ICU at 35% and entails a high degree of disability one year after assessment.
ConclusionsSevere ICH is a complex pathology that has changed some characteristics in the last two decades, with more severe patients, with more cardiovascular history and a greater predominance of brainstem and cerebellar hemorrhage. Despite the increase in severity, better parameters during the ICU stay, with open surgery used 50% less. Mortality remains stagnant at 35% with high disability per year.
Analizar el cambio de las características de presentación, evolución y tratamiento en UCI, así como la evolución funcional a los 12 meses de las hemorragias intracraneal espontáneas (HICEs) atendidas en una UCI centro de referencia.
Pacientes y métodoEstudio descriptivo, retrospectivo en Hospital de Referencia neurocríticos. Se estudian todos los ingresos de pacientes con HICE durante tres periodos: 1999–2001 (I), 2015–2016 (II) y 2020–2021 (III). Se estudian evolución en los tres periodos de variables demográficas, características basales de los pacientes, variables clínicas y características de la hemorragia, datos evolutivos en UCI. Al año valoramos la escala GOS (Glasgow Outcome Score) según presentaban mal (GOS 1−3) o buen pronóstico (GOS 4−5).
Resultados300 pacientes ingresados, distribuidos en periodos: I: 28,7%, II: 36,3% y III: 35%. 56,7% eran varones con una edad 66 (55,5–74) años; ICH score 2 (1−3). La estancia UCI fue de 5 (2–14) días con una mortalidad del 36,8%. Al año GOS 1−3 en el 67,3% y GOS 4−5 en el 32,7%. Comparando los tres periodos observamos mayor prevalencia en mujeres, y presencia de factores cardiovasculares; no cambios en la etiología; en relación a la localización, aumenta la hemorragia cerebelosa y en troncoencefalo. Aunque la gravedad fue mayor, la estancia en UCI, la utilización de ventilación mecánica invasiva y la traqueostomía son menores. La cirugía abierta ha disminuido su utilización en un 50%. La mortalidad sigue siendo alta, estancada en UCI en el 35% y conlleva un alto grado de discapacidad al año de valoración.
ConclusionesLa HICE grave es una patología compleja que ha modificado algunas características en las dos últimas décadas, con pacientes más graves, con más antecedentes cardiovasculares y mayor predominancia de la hemorragia en tronco-encéfalo y cerebelosa. A pesar de aumento gravedad, mejores parámetros en la estancia en UCI, con una cirugía abierta que se utiliza un 50% menos. La mortalidad sigue estancada en un 35% con una alta discapacidad al año.
Article

If it is the first time you have accessed you can obtain your credentials by contacting Elsevier Spain in suscripciones@elsevier.com or by calling our Customer Service at902 88 87 40 if you are calling from Spain or at +34 932 418 800 (from 9 to 18h., GMT + 1) if you are calling outside of Spain.
If you already have your login data, please click here .
If you have forgotten your password you can you can recover it by clicking here and selecting the option ¿I have forgotten my password¿.