Malignant peripheral nerve sheath tumors (MPNST) are uncommon aggressive neoplasms, frequently associated with type I neurofibromatosis. This is the first case of intradural lumbar spine MPNST with intraoperative findings of associated subarachnoid hemorrhage (SAH). A 72-year-old man presented to the emergency department with severe acute low back pain. Neurological examination was unremarkable. Gadolinium-enhanced MRI of the lumbar spine showed an irregularly shaped intradural lesion extending from L3 to L5. The lesion exhibited a medium signal both on T1 and T2-weighted imaging with peripheral enhancement. Through an L3-L5 laminectomy, a diffuse SAH, and a tumor tightly adherent to cauda equina nerve roots were found. Specimen examination revealed a fusocelular tumor with pleomorphic and hyperchromatic nuclei, positive for S100, and SOX10. On an 8-month follow-up, he had no neurological deficit, with a Karnofsky performance score of 90 points. Surgical evidence of SAH in lumbar spine intradural MPNST is a novel finding.
Los tumores malignos de la vaina del nervio periférico (TMVNP) son raros, agresivos y suelen asociarse con neurofibromatosis tipo I. Presentamos el primer caso de un TMVNP lumbar intradural con hallazgos intraoperatorios de hemorragia subaracnoidea (HSA). Un hombre de 72 años consulta a urgencias con intenso dolor lumbar, pero con examen neurológico normal. En la RM contrastada con gadolinio, se observó una lesión irregular intradural que se extendía de L3 a L5 con señal intermedia en T1 y T2, con captación de contraste periférico. Durante la laminectomía de L3-L5, se encontró HSA difusa y un tumor adherente a los nervios de la cauda equina. El espécimen tumoral mostró núcleos pleomórficos e hipercromáticos positivos para S100 y SOX10. Tras 8 meses de seguimiento, el paciente carece de déficit neurológico y puntúa 90 en la escala de rendimiento Kanofsky. La evidencia de HSA en TMVNP intradural lumbar es un hallazgo novedoso.
Malignant peripheral nerve sheath tumors (MPNST) are highly malignant soft tissue neoplasms that originate from the mesenchymal cells residing within the nerve sheath, in most cases from Schwann cells.1–3 MPNST predominantly affect young people (<40 years old), are associated with type I neurofibromatosis (NF) in 54% of cases and are commonly found in the extremities, trunk, head, and neck.1,4 Patients with MPNST have a poor prognosis with a 5-year survival rate from 16% to 44% with a local recurrence expected in 38–45% of cases. Metastases are also frequent, mainly to the lungs, liver and brain (42.3%).3–5
MPNST are a rare entity with an incidence of 0.001% that represent only 5% of all soft-tissue sarcomas.2 Spinal MPNST correspond to 2–3% of all cases, with only 6 cases of intradural lumbar MPNST reported in the literature.3 Their clinical presentation and diagnostic work-up can be misleading due to the symptomatic and imaging overlap with lumbar disc herniation.6,7 To our knowledge, this is the first case of a patient who presented with spinal subarachnoid hemorrhage (SSAH) secondary to an intradural MPNST.
Case descriptionA 72-year-old man presented to the emergency department with 2 weeks of severe lower back pain that had exacerbated over the past two days. His medical history was positive for being a heavy smoker and presenting non-quantified weight loss in the previous 6 months. The patient did not have a parent with a type 1 NF diagnosis nor any clinical criteria.8 Neurological examination was unremarkable. Complete blood count, urinalysis, and acute phase reactants were normal. Lumbar spine X-ray exhibited spondylotic changes without other important findings.
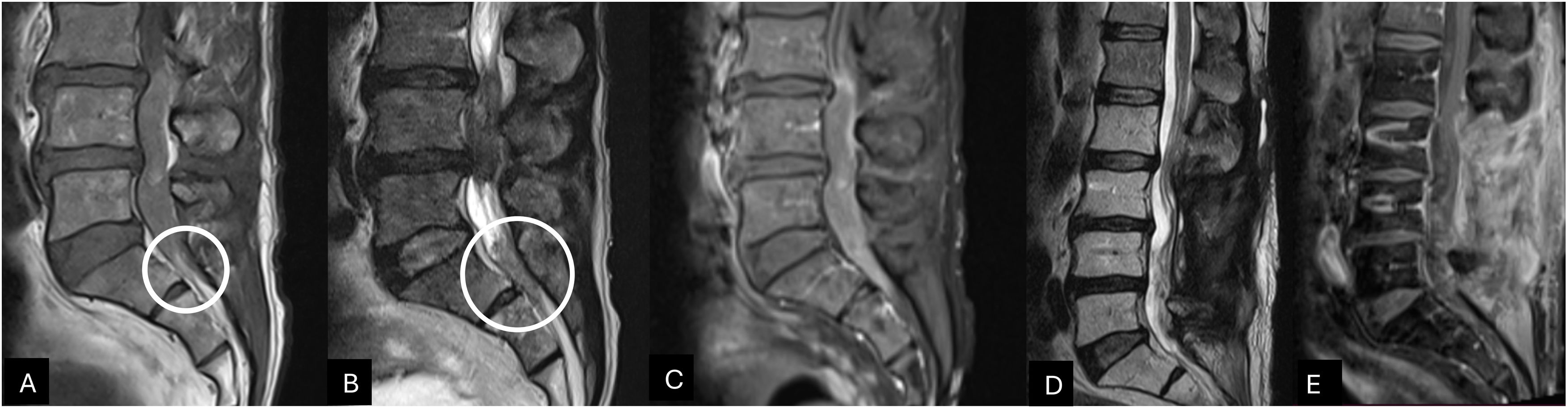
A Gadolinium-enhanced lumbar spine MRI was performed. MRI showed an irregularly shaped intradural lesion extending from L3 to L5 with a maximum length of 4.5 cm. The lesion exhibited a medium signal on T1 and hypointense on T2-weighted imaging with peripheral and irregular enhancement. A particular finding at the bottom of the thecal sac suggested subarachnoid hemorrhage according to a slightly hyperintense level on T1 and low-signal level on T2-weighted imaging corresponding to subacute bleed (Fig. 1). Spinal vascular malformation was considered as a differential diagnosis, but spinal digital subtraction angiography showed no evidence. A complete spinal MRI was performed excluding the presence of other tumors. The treatment decision was surgical resection with intraoperative neurophysiological monitoring (IONM).
Lumbar spine MRI sagittal view, T1 and T2-weighted (panel A, B) exhibiting an intradural lesion from L3 to L5 appearing isointense to CSF on T1 and hypointense on T2. On the bottom of the thecal sac, a slight hyperintense on T1 and low signal level on T2-weighted suggested subacute subarachnoid bleeding (White circle). The gadolinium-enhanced T1-weighted sagittal view (panel C) shows a peripheral enhancement of the intradural lesion.
The 8-month postoperative lumbar spine T2 and gadolinium-enhanced T1-weighted MRI sagittal views exhibited surgical and radiation therapy inflammatory changes without tumor recurrence (panels D and E).
Through an L3 to L5 laminectomy and midline durotomy, a diffuse subarachnoid hemorrhage with a xanthochromatic cerebrospinal fluid (CSF) was found. After sterile saline solution continuous irrigation, a clear CSF returned was obtained. A tumor tightly adherent to cauda equina nerve roots of approximately 3 cm was identified. Areas of highly vascular supply were found during resection. Gross total resection was achieved and IONM did not exhibit changes during tumor resection. Bone samples were sent to pathology study searching for a free margin resection, achieving samples free of tumor infiltration and confirming the GTR. Due to the risk of spinal deformity, an L3 to L5 open transpedicular fixation was completed. The patient was discharged without neurological deficit and improved pain control.
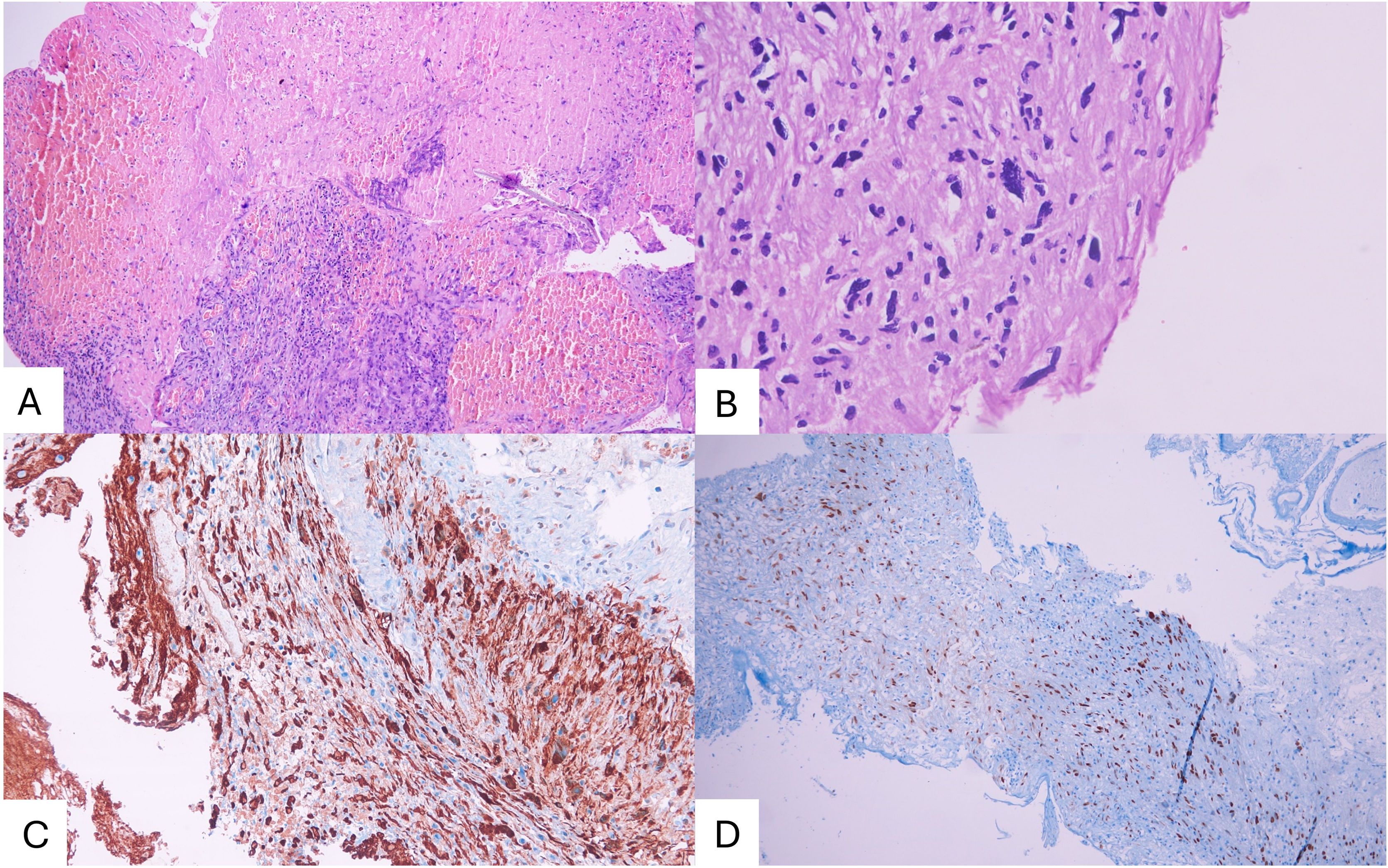
Specimen examination revealed a fusocelular tumor with pleomorphic and hyperchromatic nuclei, cytological atypia, hypercellularity, >5 mitoses/mm2, hemorrhage and necrosis. Immunohistochemical study was positive for S100 and SOX10 (Fig. 2). In addition, the Ki67 proliferative index was 15%. A diagnosis of MPNST was made. Moreover, bone and cartilage tissues were negative for malignancy. A thoraco-abdominal CT ruled out the presence of metastases. The patient underwent 25 sessions of external beam radiotherapy without adverse events with a total dose of 50 Gy. At 8-months of follow-up the patient remained without neurological deficit or pain with functional independency (Karnofsky performance score of 90 points). A follow-up lumbar spine contrast enhanced MRI showed soft-tissues radiation changes without recurrence of lesion, and the image at the thecal sac that suggested SAH disappeared (Fig. 1).
DiscussionMPNSTs are uncommon, aggressive neoplasms commonly associated with NF1 and typically present in young individuals. They represent only 5% of all soft-tissue sarcomas.3,7 In 2022, Cao, et al; performed a literature review on intradural spinal MPNST finding 55 patients. Remarkably, none of these cases had a subarachnoid hemorrhage as a surgical finding, and only in 6 patients the tumor was located at the lumbar spine.3
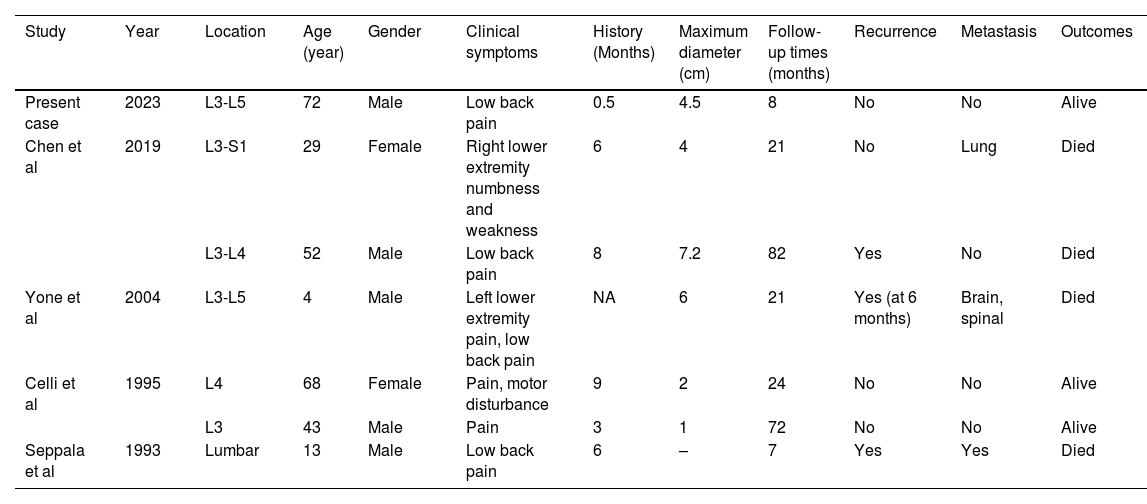
A brief summary of the patients’ characteristics with lumbar spine intradural MPNST are shown and compared in Table 1. The most frequent clinical presentation of spinal canal MPNST is characterized by local or radicular pain and motor disturbances. The mean duration from onset of symptoms to preoperative diagnosis is 12.6 months.1,3,9 On Gadolinium-enhanced lumbar spine MRI, MPNST usually appears as isointense on T1 and T2-weighted imaging, and the diameter varies from 1 to 9 cm.3,6 The irregular shape and the low level singal at the bottom of the thecal sac were signals of subarachnoid hemorrhage. Our case presents notable findings in terms of the patient's age, acute onset of symptoms, and the unique evidence of surgical subarachnoid hemorrhage (SSAH). The acute debut in our case could be explained by hemorrhage with secondary subarachnoid extension of the blood creating increase in the local pressure and roots irritation as the cause of back pain.
Demographic, clinical characteristics, follow-up and prognosis of the primary sporadic intradural MPNSTs in the lumbar Spinal canal.
| Study | Year | Location | Age (year) | Gender | Clinical symptoms | History (Months) | Maximum diameter (cm) | Follow-up times (months) | Recurrence | Metastasis | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Present case | 2023 | L3-L5 | 72 | Male | Low back pain | 0.5 | 4.5 | 8 | No | No | Alive |
| Chen et al | 2019 | L3-S1 | 29 | Female | Right lower extremity numbness and weakness | 6 | 4 | 21 | No | Lung | Died |
| L3-L4 | 52 | Male | Low back pain | 8 | 7.2 | 82 | Yes | No | Died | ||
| Yone et al | 2004 | L3-L5 | 4 | Male | Left lower extremity pain, low back pain | NA | 6 | 21 | Yes (at 6 months) | Brain, spinal | Died |
| Celli et al | 1995 | L4 | 68 | Female | Pain, motor disturbance | 9 | 2 | 24 | No | No | Alive |
| L3 | 43 | Male | Pain | 3 | 1 | 72 | No | No | Alive | ||
| Seppala et al | 1993 | Lumbar | 13 | Male | Low back pain | 6 | – | 7 | Yes | Yes | Died |
MPNST: Malignant peripheral nerve sheath tumor.
Adapted From.7 Chen J, Zheng Y, Chen Z, Fan F, Wang Y. Clinical presentation and long-term outcome of primary spinal intradural malignant peripheral nerve sheath tumors. Clin Neurol Neurosurg. 2019;185. doi:10.1016/j.clineuro.2019.105484.
Moreover, despite recent reviews on MPNST reporting clinical and radiological findings, it remains a rare entity and its diagnosis needs a thorough work-up.6 In the reported cases of MPNST by Zhao et al. and Wang et al. the initial diagnosis was a disc herniation based on symptoms, radiological findings, and surgical evidence until the pathology results were obtained.6,10 However, in our case, the diagnostic work-up lead towards a tumor etiology due to the presence of an intradural lesion with diffuse contrast enhancement, and intraoperative SSAH finding.
Among spinal hematomas, SSAH has a very low frequency.11,12 This presentation is usually related to intracranial hemorrhage secondary to trauma or aneurysm rupture, spinal tumors, and less commonly coagulopathies and spinal trauma.11 Furthermore, intraoperative evidence of SSAH is an infrequent finding during tumor removal. Kreppel et al. made a review of different types of spinal hemorrhage.12 When SSAHs was tumor-related, the main types of tumor were ependymoma, neurofibroma, and astrocytoma.12 To our knowledge, this is the first report of SSAH related to intradural lumbar spine MPNST.
Besides the scarce literature, the mainstay of treatment in MPNST is surgical resection.2,4 An aggressive resection provides local control and have been related with better outcomes.2–4 In cases involving extensive bone removal for complete tumoral resection, spinal fixation is necessary to mitigate the risk of postoperative deformity.9 Adjuvant radiotherapy has been recommended to improve local control.4 However, regardless of gross total resection and adjuvant radiotherapy, the reported 5-year survival rates vary from 16% to 44%.4 In the systematic review by Cao et al. of intradural MPNST The patients older than 30 years old and without metastases at diagnosis had better relapse-free survival and total survival period.3
ConclusionLumbar intradural MPNST is a rare etiology. The diagnosis might be challenging and initially considered in youth and NF I patients. Even lumbar disc herniation can mimic its clinical presentation. SSAH is a surgical novel finding on MPNST.
Consent to participateSigned informed consent by the patient.
Code availabilityNot applicable.
Ethics approvalInstitutional board.
Consent for publicationSigned and consented publication by the patient.
FundingNone.
Availability of data and materialNot applicable.