Nervous system involvement is uncommon in granulomatosis with polyangiitis (GPA), a systemic autoimmune disease with episodes of necrotizing vasculitis. It is usually due to the compressive effect of dural or epidural masses. Spinal hemorrhagic presentation is exceptional. A 41-year-old woman diagnosed with GPA presented with three episodes of acute spinal subdural hematoma separated by eight years and ten months, respectively. The symptomatic debut was pain and paresis in all episodes. On all occasions, a lesion compatible with acute spinal subdural hematoma was diagnosed by magnetic resonance imaging (MRI). All episodes were treated conservatively with corticosteroids and immunosuppressants. The patient presented complete neurological recovery in the first two episodes. A mild residual left lower limb paresis remains after the last one. Follow-up MRI was performed after all episodes, and no focal intraspinal lesions were detected. Spinal subdural hemorrhage is a form of manifestation of GPA, either as a debut or in the course of the disease. We describe the third confirmed case of spontaneous spinal hemorrhage secondary to GPA published in the literature and the first with recurrence. Given the extraordinary response to immunosuppressive therapy, a high level of clinical suspicion is necessary to establish treatment as early as possible.
Presentamos un caso en una paciente diagnosticada de granulomatosis con poliangeitis (GPA) que presentó en dos ocasiones separadas por un lapso de 8 años, hematoma subdural agudo espinal con diferentes niveles de afectación en cada uno de los episodios. Se añade una revisión de la literatura. Una mujer de 48 años diagnosticada de GPA, presentó tres episodios de hematoma subdural espinal agudo; separados por un período de tiempo en primer lugar de 8 años y en segundo lugar de 10 meses. Clínicamente, las tres veces se manifestaron con dolor y paresia. En todas las ocasiones, se diagnosticó mediante Resonancia Magnética Nuclear (RMN) la presencia de una lesión compatible con hematoma subdural espinal agudo. Todos los episodios se trataron de forma conservadora con corticoesteroides y Rituximab. La paciente presentó recuperación neurológica completa en los dos primeros episodios. En relación al último; presenta una paresia leve en miembro inferior izquierdo. En todos los episodios se realizó control por RMN y no se detectaron alteraciones intraespinales. La hemorragia subdural espinal es una posible forma de manifestación de la GPA, bien como debut o en el curso de la enfermedad. Es necesario un alto nivel de sospecha clínica para establecer el tratamiento de la forma más precoz posible. Presenta una respuesta extraordinaria a la terapia inmunosupresora. Es posible una recuperación completa a través del tratamiento conservador. Recomendamos un abordaje combinado.
Granulomatosis with polyangiitis (GPA, or classically, Wegener’s disease, is a rare, idiopathic, multisystemic disease mediated by an autoimmune response. The prevalence of GPA in European and American populations is about 120–140 per million. It affects men and women equally and can occur at any age but is most common in people between 45 and 60 years of age.1
Following the recommendations of several organizations, the classic name was replaced by the current and valid one, GPA, in the “2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides”.2 Although the etiology is not well established, many authors have described and expanded its pattern of involvement. First described by Klinger as a variant of polyarteritis nodosa, Friedrich Wegener first described it as an independent disease in 1936.3
GPA is one of the vasculitis included in the “small vessel” group,2,3 which contains two other diseases: microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA). GPA is also an ANCA-associated vasculitide (AAV). It is a necrotizing granulomatous inflammation with involvement mainly affecting the upper and lower respiratory tract and kidneys and necrotizing vasculitis affecting small and medium-sized vessels.
Spinal cord involvement is infrequent in GPA and the rest of AAV.4 Drachman described three mechanisms through which the nervous system may be affected in the disease: 1) systemic necrotizing vasculitis, which may involve both the central and peripheral nervous systems, predisposing to bleeding or thrombosis; 2) formation of primary necrotizing granulomas in the skull, meninges, cranial nerves, or brain; and 3) indirect damage by distant granulomatous lesions.5,6
According to the literature review, this is the third case described in the literature of spontaneous spinal subdural hematoma associated with GPA as the ultimate cause of bleeding and the first with several episodes of recurrence.
Case reportIn 2007, a late 30s woman presented with Raynaud’s phenomenon and a chronic course of recurrent vasculitis activity. The diagnosis of GPA (Wegener’s disease) was confirmed by the anatomopathological study of skeletal muscle biopsy, which confirmed the presence of necrotizing vasculitis of medium and small-caliber arterial and venous vessels in different evolutionary stages.
During her evolution, she presented focal peripheral mononeuropathy of both common peroneal nerves passing through the fibular head, skin, and joint involvement; laryngeal and tracheal involvement as well as mixed hearing loss; chronic cough with diagnosis by high-resolution computed axial tomography of thickening of the main and lobar bronchi with stenosis of the intermediate bronchus and left upper lobar bronchus. Cytologic diagnosis was negative for malignant tumor cells. Moreover, no granulomas were observed in the material obtained by broncho-aspirate. All cultures were negative. The anatomopathological study showed non-specific inflammation without conclusive signs of vasculitis. The patient underwent chronic immunosuppressive treatment with Methotrexate 15 mg weekly. Systemic outbreaks were managed with descending doses of prednisone.
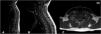
In 2012, when she was early 40s years old, she presented with sudden symptoms of paresthesia in the extremities and trunk, dorsal pain, and sensation of spinal electric shock accompanied by purplish lesions in the trunk in the absence of traumatic antecedents. Physical exam discarded any motor or sensory deficit. Magnetic resonance imaging (MRI) evidenced the presence of a spontaneous spinal subdural hematoma at C7-T4 (Fig. 1). Angiography was not performed due to the low suspicion of a cause other than her baseline disease. She was treated conservatively with corticosteroids and Cyclophosphamide. Follow-up MRI showed the resolution of the spinal subdural hematoma and ruled out the presence of vascular malformations at all spinal levels.
First episode (November 2012). (A and B) Sagittal MRI, T1-weighted sequence; (C) Axial MRI, T1-weighted sequence. Intra-spinal lesion compatible with hematoma (red arrow); it is hyperintense on T1-weighted sequence with anterior and right lateral subdural location, cranial limit slightly above T1 and slightly lower caudal T5. It originates medullary displacement towards the left lateral region.
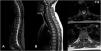
In March 2020, during a quiescent period of the disease and under Methotrexate 15 mg weekly, she went to the Emergency Department referring weakness in the right lower limb, paresthesia in both feet and hands as well as the presence of sudden cervical pain radiating to the suboccipital region of two days of evolution. In addition, she reported worsening basal paresthesia in the extremities, becoming continuous and of greater intensity. Physical exam evidenced right hemiparesis, hypoesthesia, and allodynia. A spinal MRI showed an intrathecal hematoma in the subdural space. The spinal cord and conus medullaris preserved standard thickness and signal intensity (Fig. 2). Laboratory tests also confirmed normal parameters; erythrocyte sedimentation rate and C-reactive protein were normal; the ANCA profile showed positivity for p-ANCA and Anti-MPO, with negative values for c-ANCA and Anti-PR3.
Second episode (March 2020). (A-B-C) Sagittal MRI, T1-weighted sequence; (C) Axial MRI, T1-weighted sequence. Visualization of intra-spinal hematoma in subdural location, only visible in the T1-weighted sequence where it presents isointense signal behavior compared with the spinal cord (red arrow), observing obliteration of the subarachnoid space from the clivus to T1 (anterior) and from C3–C4 to T1 (anterior and posterior).
A combined approach between Neurosurgery and Rheumatology recommended conservative management with the same corticosteroid regimen used in the previous episode of spinal bleeding (three daily boluses of methylprednisolone 750 mg followed by prednisone 60 mg), as well as the use of a soft collar for the sitting and standing position. Rituximab was prescribed as a subsequent corticosteroid-sparing treatment. However, the administration was delayed due to a higher risk of severe disease in case of COVID-19 infection.
In December 2020, she consulted again for sudden pain in the left iliac fossa with bilateral irradiation. She also referred to left hypoesthesia at the T7 level and perineal region. Subsequently, she had difficulty ambulating due to weakness in the left lower limb. Spinal MRI showed a subdural hematoma from T3 to T10 (Fig. 3).
Third episode (December 2020). (A) Sagittal MRI, T2-weighted sequence; (B) Sagittal MRI, T1-weighted sequence; (C) Axial MRI, T2 (superior) and T1(inferior) -weighted sequences. Extra-axial occupancy suggestive of subdural hematoma from T3 to T10 (red arrow), consisting of heterogeneous hypointense signal in T2-weighted sequences and discrete hyperintensity in T1-weighted sequences.
Conservative treatment was decided again (dexamethasone initially and prednisone subsequently). Likewise, immunosuppressive treatment with Rituximab was reinitiated. The motor weakness improved, and the patient presents minimal residual paresis, predominantly proximal in the left lower limb, accompanied by left hypoesthesia at the left costal and abdominal region.
Follow-up MRI in February 2021 showed complete resolution of the intrathecal hemorrhage. Since then, the patient has had no new outbreaks and currently undergoes therapy with prednisone, methotrexate, and rituximab. Fig. 4 summarizes the evolution of the disease in this patient.
DiscussionWe describe an exceptional case of a patient with a previous diagnosis of GPA presenting with three independent episodes of spontaneous spinal subdural hematoma involving different spinal segments. Despite intrathecal bleeding being rare, the meningeal involvement in the context of underlying vasculitis has been postulated as the mechanism of action. Conservative management is valid and effective in cases of mild non-progressive neurological deficit.
Several authors have described spinal cord compression due to extradural and subdural granulomatous proliferation in patients with GPA.5–8 However, only two cases of spontaneous spinal hemorrhage have been published to date in the context of the disease (Table 1).9,10
Summary of patients with spontaneous spinal subdural hematoma and GPA reported in the literature.
| Author (year) | Sex | Age | Location | Surgery | Medical treatment | Neurological outcome |
|---|---|---|---|---|---|---|
| Guilfoyle et al. (2010)9 | F | 21 | Thoracic (T1–T4) | Yes | CS + MMF | Remission |
| Kamo et al. (2012)10 | F | 75 | Lumbosacral (L2–S3) | No | Not specified | Remission |
| Present case, (2023) | F | 41 | Cervicothoracic (C7–T4) | No | CS + CPA | Complete recovery |
| 48 | Cervicothoracic (clivus-T1/C3–C4-T1 | No | CS + RTX | Complete recovery | ||
| 49 | Thoracic (T3–T10) | No | CS + RTX | Remission (mild paresis) |
Abbreviations: CS: corticosteroids; CPA: cyclophosphamide; MMF: mycophenolate mofetil; RTX: rituximab.
Anatomically, the subdural space is potentially avascular. Kobayashi et al.11 postulate that the primary origin of the bleeding comes from the subarachnoid or epidural space. This theory supports that the bleeding comes from the former, as the arachnoid is a weaker and more porous layer than the dura mater.
Guilfoyle et al.9 reported a patient diagnosed with GPA with posterior extramedullary intradural bleeding between the first and fourth thoracic segments, compatible with bleeding that would cause spinal cord compression and paraplegia. Surgical evacuation confirmed an acute hematoma without associated vascular malformations in the subdural and subarachnoid space. The second patient, described by Kamo et al.,10 presented a spontaneous lumbar hematoma initially misdiagnosed with epidural lipomatosis and managed conservatively. The history of GPA, the detailed analysis of the MRI, and the clinical-radiological evolution led to suspect a spontaneous lumbar hematoma.
Most spinal hemorrhages considered “spontaneous” result from vascular malformations or trauma in the presence of coagulopathy; truly idiopathic cases are rare.12–14 Domenicucci et al.13 reported an extensive series (n = 106), including those of iatrogenic cause. Later, Beer et al.14 analyzed the largest series (n = 122) of spontaneous, non-iatrogenic spinal subdural hematomas. In their analysis, only 5 cases were due to vasculopathy (4%), an etiology not previously recognized as a possible cause of spontaneous spinal bleeding. In detail, they described primary central nervous system vasculitis (n = 1), EGPA (n = 2) and GPA (n = 2).
Most spinal hematomas are characterized by an acute or subacute course with sudden onset of pain at the level of bleeding and symptoms of spinal cord compression of varying intensity (loss of strength or sensation), with or without bladder/intestinal alterations.9,10,12–14 The thoracic spine is the most affected, followed by the thoracolumbar and the cervicothoracic spine.14 Early diagnosis and treatment are essential since the reversibility of neurological damage depends on it.11,12 The patient hereby reported presented in the three episodes with pain and mild paresis. The affected level differed on each occasion: upper dorsal spine, cervicodorsal junction, and mid-lower thoracic segment. Given the multilevel involvement in episodes separated in time, the history of PGA, and the absence of structural lesions in control MRI studies, it seems reasonable to consider PGA the most likely cause of the bleeding.
Venning et al.15 postulated that some patients with subarachnoid hemorrhage and negative four-vessel angiography may result from occult central nervous system vasculitis, and the small vessels involved in GPA are often below the sensitivity of routine arteriography. However, the incomplete diagnostic study with angiogram is one of the most relevant limitations in the case described since this is the gold standard test. The study should have been performed at least after a second bleeding episode. Nevertheless, the exceptional situation of COVID-19 pandemic prevented a complete diagnostic study.
Conservative non-surgical management is justified if the neurological deficit is minor,12 as the excellent response of GPA to immunosuppressive therapy has been widely described in the literature.3,4,6 However, in case of significant neurological deficit or rapid clinical or radiological deterioration, surgical treatment should be indicated, consisting of early decompressive laminectomy with the evacuation of the subdural hematoma (with or without additional fusion, depending on the characteristics of each patient).
The pharmacological treatment of GPA consists of two phases: a first induction phase aimed at achieving remission and a second maintenance phase to consolidate remission and avoid relapses. High-dose corticosteroids associated with another immunosuppressant (Cyclophosphamide or Rituximab) are recommended for induction in the systemic form of the disease. Some cases may require plasmapheresis.7,11 That combination has proven effective but not as safe for patients because of side effects. Zheng et al.4 and Tavakolpour et al.16 evidenced how Rituximab is safer than Cyclophosphamide and non-inferior to induce remission of a GPA outbreak. It is also significantly safer than Azathioprine for use in the maintenance phase. Thus, Rituximab is recommended based on at least similar efficacy with a much higher safety profile. Rituximab was prescribed to the patient hereby reported after the second bleeding episode. However, the treatment was delayed because of the possible poor association evidenced in clinical practice with more severe and persistent COVID-19 pneumonia and increased risk of reinfection.17–22
ConclusionGPA is a multiorgan disease that can cause alterations through several mechanisms. Spinal subdural hemorrhage is rare, either as a debut or during the disease. A high level of clinical suspicion is necessary to diagnose and establish the optimal treatment as early as possible. It presents an extraordinary response to immunosuppressive therapy, making possible a complete recovery through conservative treatment. However, surgery is necessary in some cases of severe neurological deficit or rapid deterioration, so multidisciplinary management is essential.
FundingNone of the authors have received any financial support for this study.
The authors report no conflict of interes