Deep brain stimulation (DBS) of the subthalamic nucleus (STN) is a recognized treatment for drug-refractory Parkinson's disease (PD). However, the therapeutic success depends on the accuracy of targeting. This study aimed to evaluate potential accuracy differences in the placement of the first and second electrodes implanted, by comparing chosen electrode trajectories, STN activity detected during microelectrode recording (MER), and the mismatch between the initially planned and final electrode positions on each side.
Materials and methodsIn this retrospective cohort study, we analyzed data from 30 patients who underwent one-stage bilateral DBS. For most patients, three arrays of microelectrodes were used to determine the physiological location of the STN. Final target location depended also on the results of intraoperative stimulation. The choice of central versus non-central channels was compared. The Euclidean vector deviation was calculated using the initially planned coordinates and the final position of the tip of the electrode according to a CT scan taken at least a month after the surgery.
ResultsThe central channel was chosen in 70% of cases on the first side and 40% of cases on the second side. The mean length of high-quality STN activity recorded in the central channel was longer on the first side than the second (3.07±1.85mm vs. 2.75±1.94mm), while in the anterior channel there were better MER recordings on the second side (1.59±2.07mm on the first side vs. 2.78±2.14mm on the second). Regarding the mismatch between planned versus final electrode position, electrodes on the first side were placed on average 0.178±0.917mm lateral, 0.126±1.10mm posterior and 1.48±1.64mm inferior to the planned target, while the electrodes placed on the second side were 0.251±1.08mm medial, 0.355±1.29mm anterior and 2.26±1.47mm inferior to the planned target.
ConclusionThere was a tendency for the anterior trajectory to be chosen more frequently than the central on the second side. There was also a statistically significant deviation of the second electrodes in the anterior and inferior directions, when compared to the electrodes on the first side, suggesting that another cause other than brain shift may be responsible. We should therefore factor this during planning for the second implanted side. It might be useful to plan the second side more anteriorly, possibly reducing the number of MER trajectories tested and the duration of surgery.
La estimulación cerebral profunda (ECP) del núcleo subtalámico (NST) es reconocida como un tratamiento para la enfermedad de Parkinson (EP) refractaria al tratamiento farmacológico. Sin embargo, el éxito de esta intervención depende de la precisión de la colocación de los electrodos. Este estudio tuvo como objetivo evaluar las posibles diferencias de precisión entre la colocación del primer y segundo electrodo, comparando las trayectorias elegidas para cada lado, la actividad del NST detectada durante el microrregistro (MER) y la discrepancia entre las posiciones inicialmente planeadas y las finales.
Materiales y métodosEn este estudio retrospectivo analizamos datos de 30 pacientes sometidos a ECP bilateral. En la mayoría de los casos se usaron tres conjuntos de microelectrodos para determinar la ubicación fisiológica del NST. El posicionamiento final del electrodo estuvo asimismo condicionado por los resultados de la estimulación intraoperatoria. Se comparó la elección de canales centrales vs. no centrales. El vector euclidiano del desvío se calculó a partir de las coordenadas planeadas inicialmente y la posición final de la punta del electrodo, según una tomografía computarizada realizada al menos un mes después de la cirugía.
ResultadosLa trayectoria central se eligió en 70% de los casos en el primer lado y en el 40% de los casos en el segundo lado. La duración media de la actividad de alta calidad del NST registrada en el canal central fue mayor en el primer lado que en el segundo (3,07±1,85mm vs. 2,75±1,94mm), mientras que en el canal anterior hubo mejores registros de MER en el segundo lado (1,59±2,07mm en el primer lado vs. 2,78±2,14mm en el segundo). En cuanto a la discrepancia entre la diana planeada y la posición final de los electrodos, los electrodos del primer lado se colocaron, por término medio, 0,178±0,917mm laterales, 0,126±1,10mm posteriores y 1,48±1,64mm inferiores al objetivo, mientras que los electrodos colocados en el segundo lado estaban 0,251±1,08mm mediales, 0,355±1,29mm anteriores y 2,26±1,47mm inferiores al objetivo planificado.
ConclusiónEn el segundo lado, observamos una tendencia a elegir la trayectoria anterior mas frecuentemente que la central. También hubo un desvío estadísticamente significativo de los segundos electrodos, en dirección anterior e inferior, en comparación con los electrodos del primer lado, lo que sugiere que la causa puede ser otra que no el brain shift. Por lo tanto, debemos tener esto en cuenta al planear la inserción del electrodo en el segundo lado. Podría ser útil planear el segundo lado más anteriormente, posiblemente reduciendo el número de trayectorias probadas por MER y la duración de la cirugía.
Deep brain stimulation (DBS) is an established treatment for advanced, drug-refractory Parkinson's disease (PD), comprising the application of chronic high-frequency electrical stimulation through an electrode implanted in a specific brain target.1,2 It produces a superior improvement in quality of life and reduction of motor symptoms’ severity than isolated pharmacological therapy.3,4 A commonly chosen target in PD is the subthalamic nucleus (STN).
The therapeutic success of DBS depends on the accuracy of STN targeting.5–7 Literature shows that it is essential to target the dorsolateral, motor subdivision of the STN, avoiding penetration of its limbic and associative subdivisions (which might lead to executive function deficits and mood impairment), or the laterally located internal capsule (which could cause tonic muscular contractions and slurred speech).1,8 Thus, accurate STN targeting requires rigorous care at different steps, including planning and surgical execution.
However, stereotactic neurosurgery relies on the assumption that brain structures are static while events such as brain shift can hinder the correct placement of the electrodes, causing a discrepancy between the images acquired preoperatively and the actual location of structures during and after surgery.8,9 This movement of the brain is due to various factors, particularly the force of gravity, and the invasion of the intracranial space by air and the loss of cerebrospinal fluid that occur during the opening of the dura mater, causing an anterior pneumocephalus.10–12 This explains why the shift generally occurs in a posterior direction when the patient is placed in a supine position during surgery.13,14 While some studies argue that shift of subcortical structures is very limited and does not significantly affect clinical outcome,10,15 Hunsche et al. defend that in bilateral DBS the brain shift that occurs after placing the first electrode should be considered relevant as it can affect the placement of the second one.16 Other studies have also shown that more adjustment with MER and intraoperative macrostimulation is usually required for the correct placement of the second electrode.6,17 It is therefore possible to have a more significant shift (thus increasing the distance between the planned target and the final electrode location) on the second implanted side, when compared to the first.7 The reduced accuracy in placement of the second electrode is related to a reduction in the threshold for adverse effects, and a less pronounced improvement in symptoms.18,19
Taking this into account, this study aims to evaluate potential differences in targeting accuracy (defined as final electrode position equal to initially planned target), comparing the first and second implanted sides in patients undergoing bilateral STN-DBS surgery for PD. We hypothesize that if there are no differences in targeting accuracy between the first and second sides, then the frequency of choosing the central channel for final implantation will be similar for both. However, in case there are in fact differences, then this should translate into a distinct quality of intraoperative electrophysiological recordings, with an expected higher quality of STN signal detected in the central channel of the side with the better targeting accuracy (in this case, the first side). In this situation, another expected observation is a different magnitude of mismatch between the initially planned versus final electrode positions, with a greater deviation on the second side.
Materials and methodsStudy populationThis observational retrospective cohort study included all consecutive patients who underwent simultaneous bilateral DBS surgery for the treatment of PD, with the STN as the target, from January 2019 to February 2021, and who had at least one month of follow-up. Patients who did not have a CT scan taken at least a month after the surgery, or who lacked data about the initial stereotactic planning, were excluded from this analysis. Patient data was collected from medical records in our database. The research protocol was approved by the ethics committee of the São João Hospital Centre.
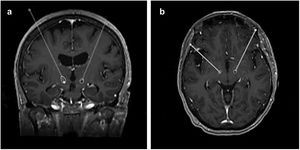
Surgical procedureStereotactic planning was done by fusing a preoperative 1.5T or 3T MRI with a stereotactic CT scan obtained on the morning of the surgery, using FrameLink StealthStation 8® (Medtronic, USA), as previously described.20 After verification of fusion accuracy, the mid AC-PC point was used to locate the anatomically defined target (12mm lateral, 2mm posterior and 4mm inferior). The final position of the target was confirmed through direct visualization of the STN in T2-weighted or T2 SPACE images, followed by manual adjustment.
The surgery was performed with the patient supine, with a 30° head flexion, and under local anaesthesia. The first side operated was always contralateral to the most symptomatic side.
Whenever possible, three steel cannulas and microelectrodes (central, anterior and lateral) were inserted to perform stimulation. MER was started 5mm above the planned target, advanced in 0.5mm steps, and extended 3–5mm below the target point or until the substantia nigra was detected, as previously described.21 Two experienced neurologists performed a visual and sound analysis of the single unit recordings captured by the high impedance electrodes, and qualitatively scored each 0.5mm step using the following scale: 0 (no signal), 1 (low-quality: only traces visible), 2 (medium-quality: sparse activity) or 3 (high-quality: abundant STN-typical spikes). The STN is characterized by a mean firing rate of 37±17Hz, large amplitude of spikes and irregular rhythm.22 We used this information to estimate the length of high-quality STN activity detected along each channel. In case of poor MER results, lack of motor benefit, or presence of adverse effects in the original three trajectories (central, anterior and lateral), 2 additional cannulas in the medial and posterior channels were implanted in order to probe the quality of these regions. The channel and the final depth of implantation (defined as position of the lowermost contact) of each electrode were decided based on MER and macrostimulation test results. In our cohort, most patients received Activa™ PC or Percept™ PC (Medtronic, USA) IPGs, while a few received Vercise™ PC (Boston Scientific, USA) IPGs.
After the surgery, a CT scan was performed and fused with the preoperative MRI to evaluate the final position of the electrodes and exclude complications such as haemorrhage or ischaemia. In this study, this same analysis was repeated at least a month after the surgery, making use of the FrameLink StealthStation 8® software to determine the final coordinates of the tip of the electrode (shown in Fig. 1).
Statistical analysisStatistical analysis and graphs were performed using GraphPad Prism (Version 9.0.0, GraphPad Software, San Diego, California, USA) and IBM SPSS Statistics for Windows (Version 27.0, IBM, Armonk, New York, USA). The Kolmogorov–Smirnov test was used to assess if the data had a normal distribution. McNemar's test was used to analyze differences in the chosen electrode trajectory (central vs. non-central) between the first and second sides, and the left and right hemispheres. Using the initially planned coordinates from saved surgery plans, and the final electrode locations, the Euclidean vector deviation was calculated using Pythagoras’ theorem applied to three-dimensional space. Displacements in the x-, y- and z-directions were calculated by subtracting final and planned coordinates in each respective plane. Paired t-test was then used to evaluate differences between the first and second sides. Regarding the length of high-quality STN activity on each channel, comparisons were done using the Wilcoxon matched-pairs signed-rank test. Categorical variables were expressed as percentages and continuous variables as mean±standard deviation. The level of significance considered was p<0.05.
ResultsStudy populationOf the 52 patients who received bilateral DBS surgery between January 2019 and February 2021, only 30 patients (60 implanted electrodes) fulfilled all the criteria and were included in this study.
Sixteen (53%) of the patients were female, the mean age at the time of surgery was 62.43±6.63 years and the mean duration of disease was 11.3±5.74 years. The mean UPDRS-III score off medication before surgery was 54.6±14.8. One month after surgery, the mean UPDRS-III score off medication and with stimulation switched on was 13.5±7.49. Sixteen patients (53%) had the first electrode implanted on the right side (shown in Table 1).
Patient demographics.
| Number of patients | 30 |
| Female patients, n (%) | 16 (53%) |
| Age at the time of surgery, mean years±SD | 62.4±6.63 |
| Duration of disease, mean years±SD | 11.3±5.74 |
| Patients with right side as first implanted side, n (%) | 16 (53%) |
| UPDRS-III med-off before surgery, mean±SD | 54.6±14.8 |
| UPDRS-III med-off 1 month after surgery, mean±SD | 13.5±7.49 |
Overall, the central channel was the most often used, in a total of 33 electrodes (55%), followed by the anterior channel, chosen for 21 electrodes (35%). The lateral channel was used in 4 cases and the medial in 2. The trajectory used for the implantation of the electrodes was symmetrical in 43% of patients.
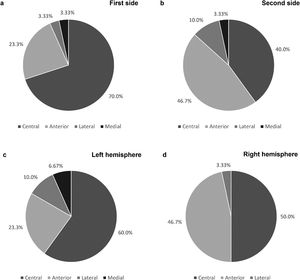
As shown in Fig. 2a and b, the final leads were implanted in the central channel more frequently on the first side: in 21 patients (70.0%) for the first side and in 12 patients (40.0%) for the second (p=0.022). Furthermore, regarding the first implanted side, the anterior channel was chosen in 7 patients (23.3%), while the lateral and medial channels were chosen in only 1 patient each. Concerning the second side, the anterior channel was used in 14 patients (46.7%), the lateral in 3 (10.0%) and the medial in 1 (3.33%). Cases where the medial channel was chosen were due to the low threshold for adverse effects in the other channels. The posterior channel was never used on either side.
When comparing the electrode trajectories chosen on the left and right cerebral hemispheres, regardless of which side was first implanted, the central channel was chosen in 60% and 50% of the cases, the anterior in 23.3% and 46.7%, and the lateral in 10.0% and 3.33%, respectively. The medial channel was only used in the left hemisphere. The differences between both hemispheres were not statistically significant (p=0.58; shown in Fig. 2c and d).
STN activity detected by MERRegarding the first side, MER activity was observed in at least one channel in all patients; however, there were 5 patients where not all channels were tested (1 anterior, 3 lateral, and 1 anterior and lateral). On the second side, all channels were tested in every patient, but there were 3 cases where there was no high-quality MER activity in any of them. In these situations, the final trajectories chosen were based on the macrostimulation test results alone.
The mean length of high-quality STN activity recorded in each channel is shown in Table 2. For the central channel, the first side showed a longer length of activity than the second, but the difference wasn’t significant (3.07±1.85mm vs. 2.75±1.94mm; p=0.539). On the other hand, the second side showed a significant increase in activity on the anterior channel when compared to the first (2.78±2.14mm vs. 1.59±2.07mm, respectively; p=0.0367). In the lateral channel, both sides had a similar length of STN activity (2.10±1.94mm on the first side vs. 2.15±1.97mm on the second; p=0.775). However, the final trajectory chosen did not always coincide with the channel that showed the longest MER activity, as this decision was also influenced by the results of intraoperative macrostimulation. The final trajectory chosen corresponded with the one with better MER results in 19 cases (63%) on the first side and 17 cases (57%) on the second side.
High-quality electrophysiological activity of the STN recorded during MER.
| First side | Second side | p-Value | |
|---|---|---|---|
| Central channel, n | 30 | 30 | |
| Mean length, mm±SD | 3.07±1.85 | 2.75±1.94 | 0.539 |
| No STN activity, n (%) | 5 (17) | 7 (23) | |
| Anterior channel, n | 28 | 30 | |
| Mean length, mm±SD | 1.59±2.07 | 2.78±2.14 | 0.0367 |
| No STN activity, n (%) | 14 (50) | 9 (30) | |
| Lateral channel, n | 26 | 30 | |
| Mean length, mm±SD | 2.10±1.94 | 2.15±1.97 | 0.775 |
| No STN activity, n (%) | 10 (38) | 11 (37) | |
| Extra channels | |||
| Medial channel, n (%) | 1 (3) | 1 (3) | |
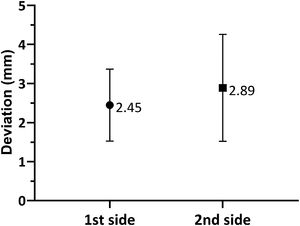
As shown in Fig. 3, when comparing the 3D Euclidean vector deviation, regardless of the direction of movement, there was no statistically significant difference between both sides (2.45±0.921mm on the first side vs. 2.89±1.37mm on the second side; p=0.0881). However, when comparing separate displacements in the x-, y- and z-directions, we found that electrodes implanted on the first side were placed on average 0.178±0.917mm lateral, 0.126±1.10mm posterior and 1.48±1.64mm inferior to the planned target, while the electrodes placed on the second side were 0.251±1.08mm medial, 0.355±1.29mm anterior and 2.26±1.47mm inferior to the initial target (shown in Fig. 4a and b). The differences in deviation in the y-direction and z-direction were statistically significant (p=0.0443 and p=0.0029, respectively), unlike those in the x-direction (p=0.104).
Our results showed a tendency to opt for the central channel more frequently on the first side than on the second. This difference was not attributable to a specific brain hemisphere, but rather to the order by which the electrodes were implanted. Two studies reported a similar trend, with the central channel being used more on the first side than the second, while the opposite occurred with the anterior channel.21,23 Others showed that, despite the central trajectory prevailing on both sides, the percentage of cases where the anterior trajectory was chosen was higher on the second side.24–26 Conversely, in a study by Umemura et al., 21% of the electrodes were placed in a trajectory other than the central. In these cases, the posterior channel was the most frequently chosen, followed by the anterior channel.27 In another study, MER results led to a medial and posterior correction of 35.7% of the original stereotactic coordinates obtained by CT-MRI image fusion.28 In these studies, brain shift played a role in the differences observed.
STN activity detected by MERIn line with our initial hypothesis, the mean length of high-quality STN activity detected in our cohort was larger in the central channel on the first side, while in the anterior channel, better recordings were obtained on the second side. This supports the case for a lower accuracy in the final positioning of the second implanted electrode, in relation to the initially planned target.
Planned versus final electrode position mismatchOur results showed no statistically significant differences regarding the Euclidean vector displacement, which is in agreement with results from other authors.6,10 Nevertheless, when analysing displacement in each axis, we found a tendency for the second electrode to be positioned more anterior and inferior to the planned target than the first one, further supporting our hypothesis. Other studies reported similar results.6,7,18 Slotty et al. also showed that the highest median deviation on both sides was seen in the z-axis as it is prone to larger changes depending on the results of MER.10 In our cohort, the larger deviation in the z-axis is probably due to the use of directional leads, which must be implanted at a greater depth than the one initially planned to ensure that contacts are placed on the target. Still, it should be emphasized that changes in the depth of the electrode are not equal to changes in the z-axis. Movement along the trajectory of the electrode will affect the target position in all axis, depending on the angle of insertion.17
Brain shift has been appointed as a cause for the differences observed between the first and second operated sides. According to this hypothesis, one would expect the STN on the second side to shift posteriorly and, therefore, lower quality MER activity on the anterior channel and, consequently, this trajectory to be chosen less frequently. However, our results show exactly the opposite pattern, which is in line with studies that have shown that brain shift in subcortical structures is very limited.10,15 Supporting the external validity of our data, two other studies have reported similar results, with an increased use of anterior trajectories on the second side.21,23 However, they offer no probable explanation for this difference. Chrastina et al. reported that, despite the central trajectory prevailing on both sides, the anterior trajectory was still chosen more frequently on the second side than on the first. They suggest that causes other than brain shift must play a role.24
Consequently, other factors beyond pneumocephalus should be taken into consideration. A hypothesis is image distortion due to MRI, since it does not provide an accurate representation of the electrophysiological boundaries of the STN, therefore we should not rely solely on this information. For this reason, we fused MRI and CT images. However, since the pre-operative MRI is done with the patient supine, the true position of the STN during surgery might no longer be accurately represented by the planned target, as the patient is placed at a 30° angle. Additional sources of error include imaging discrepancies, errors in target selection, and vector calculations.8 Surgical factors, such as dislocation or mechanical inaccuracy of the stereotactic frame, misinterpretation of the MER data, or factors related to the patient or the inherent disease, such as age or disease duration, can also play a role in these alterations.24
Study limitationsThe present study has some limitations. First, its retrospective nature and the small sample size mean that any extrapolations from our findings should be taken with caution, although the high consistency between our electrophysiological and anatomical approaches provides an important degree of robustness to our conclusions. Second, the identification of the final electrode position was done manually, by direct visualization in post-operative CT scans. This too can be a source of error, as the diameter of the leads is 1.27mm, but the artefact can measure up to 3.3mm, hindering the identification of the centre of the most distal tip of the lead.29 Furthermore, the CT scan images used have a slice thickness of 1mm so any values below this cannot be considered accurate. Third, the stereotactic techniques used can be responsible for a Euclidean distance of up to 2mm between the planned target and the final lead location,8 though more recent methods managed to obtain deviations smaller than 1.5mm.30 Our results fit this range of values, despite being larger than those obtained by some other studies.6,31
Future studies should be conducted to compare electrode displacement between the planned target and the electrode artefact in immediate and in delayed post-operative images, as well as to evaluate the clinical significance of the differences observed, by comparing the preoperative and postoperative UPDRS-III scores on each implanted side.
ConclusionThis study showed that in bilateral STN-DBS there is a tendency for the placement of the first implanted electrode to be more accurate and for the second electrode to have a statistically significant deviation in the anterior and inferior directions. The initial target planning could possibly benefit from previously placing the second electrode more anteriorly, therefore reducing the number of MER trajectories tested and, hence, the duration of the surgery. Additionally, these results suggest that brain shift cannot be considered the main cause for the differences observed in our cohort. Further studies, with larger sample sizes and prospective designs, are required to analyze the role played by other factors. Nevertheless, our findings should be taken into account during planning for the second implanted side, in order to achieve greater accuracy in the placement of electrodes.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNothing to declare.
The authors wish to thank the Neurosurgery and Neurology teams of the São João Hospital Centre, in particular the members of the Movement Disorders and Functional Neurosurgery Unit.